A metal alloy that is liquid at room temperature.
Suppose you had a metal alloy that had the advantages of liquid mercury, but without the toxic effects?
You could make your own barometers and thermometers, and not worry about calling in a hazardous materials team to clean up after any accidents. You could simply wipe up the mess with a paper towel. You wouldn't have to worry about breathing in toxic mercury fumes, but you could still make neat little electric motors that dip into liquid metal to make their electrical connections.
Suppose further, that the metal would stick to glass, so you could paint it on glass to make your own mirrors. Or that it would stick to paper so you could draw your own electric circuits in it?
Fun things to do with liquid metal
One fun thing you can do right away with the liquid metal alloy is make your own mirrors. All it takes is a piece of glass and a cotton swab.

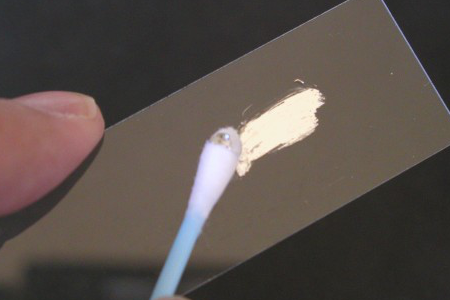
Now rub the coated swab on the glass (in the phot we are using a glass microscope slide). The metal sticks to the glass, and makes an opaque reflective coating.
More fun things
There are lots of things you can do with liquid metal:
- Make thermometers
- Make barometers
- Make tilt meter seismographs
- Make non-conductive objects conductive
- Make electrodes that conform to varying surfaces
- Experiment with magnetohydrodynamics
- Wiggle it with high frequency electricity
- Use it to conduct high energy sound
- Replace mercury in spinning telescope mirrors
If you need a shiny surface, a dilute solution of hydrochloric acid can be placed on the surface, or you can use a light coating of mineral oil. Both will prevent the slow oxidation of the metal that occurs over time.
How does it do that?
Gallium is an element (atomic number 31, right below aluminum and just to the right of zinc in the periodic table of the elements). It starts out with a very low melting point already, but we can add some other elements to get an even lower melting point.
Right below gallium in the periodic table is indium (element 49). Just to the right of indium is tin (element 50).
When these elements are combined, their atoms bind together into a compound. The molecules of that compound do not bind to one another as much as the atoms of the original metals bound to each other. This lowers the melting point.
There are many ways to combine the three metals:
|
Compound |
Percentages |
Grams Ga |
Grams In |
Grams Sn |
|
Ga14In3Sn2 |
62.65% Ga, 22.11% In, 15.24% Sn |
97.6122 |
34.4454 |
23.742 |
|
Ga17In4Sn2 |
62.98% Ga, 24.40% In, 12.62% Sn |
118.529 |
45.9272 |
23.742 |
|
Ga22In5Sn3 |
62.25% Ga, 23.30% In, 14.45% Sn |
153.391 |
57.409 |
35.613 |
|
Ga25In5Sn4 |
62.43% Ga, 20.56% In, 17.01% Sn |
174.308 |
57.409 |
47.484 |
|
Ga25In6Sn3 |
62.52% Ga, 24.71% In, 12.77% Sn |
174.308 |
68.8908 |
35.613 |
Each combination will have a slightly different melting point. Which do you think has the lowest melting point? This might make a good science fair experiment.
A mixture of 76% gallium and 24% indium melts at 16° Celsius (61° Fahrenheit). Both gallium and this combination can be supercooled. That means that once melted, they can stay liquid even though they are cooled well below their melting points. Eventually a small crystal forms, and starts the whole batch solidifying, but small amounts can be kept supercooled for quite a while.
The gallium-indium alloy is more reflective than mercury, and is less dense, so it is being explored as a replacement for mercury in spinning liquid mirrors for astronomical telescopes.
Product recommendation:Low Melting Point Alloys
Changsha SANTECH provides Low Oxygen Type Indium Alloys,Low Oxygen Type Gallium Alloys,Gallium Indium Tin Alloy. Please email us if you are interested in our products. Thank you!

 EN
EN  AR
AR FR
FR DE
DE JA
JA PT
PT CN
CN